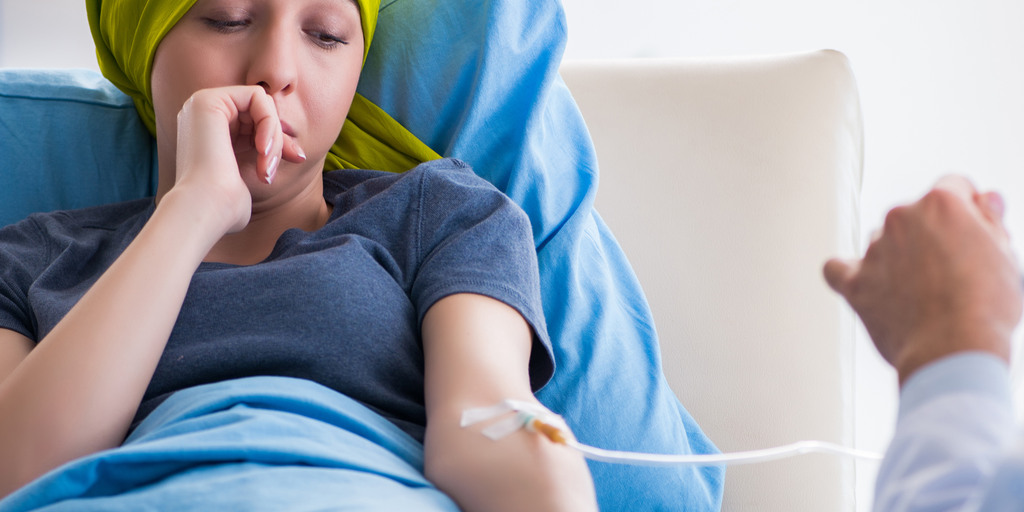
Advancements in global medicine and specialist care are essential to improving quality of life, treatment options, and survival rates for those with a cancer diagnosis, as well as mitigating the side effects that come with receiving those treatments. Recently, the US Food and Drug Administration (FDA) approved for marketing Ayana’s Doxorubicin-HCI Liposomal Injection, an Israeli groundbreaking generic treatment for ovarian cancer, multiple myeloma and Kaposi’s Sarcoma. This drug aims to alleviate the side effects of cancer treatment as it directly targets the cancer-infected cells. As a chemotherapy drug, the treatment uses cytotoxin and is compressed in a closed lipid sphere called a liposome. The targeted therapy aims to mitigate one of the challenges of chemo: the side effects, reducing hair loss, gastrointestinal issues, and other adverse effects.
In January 2019, CSOFT published a blog on Reducing the Side Effects of Chemo for Cancer Patients, which explored how a new cancer treatment device is helping to reduce the negative effects of chemotherapy on cancer patients. As the blog explains, in general, when chemotherapy is injected into the human body, the chemicals target and destroy the active cancer cells. The intended target, of course, is the tumor but unfortunately, a large amount of the chemical infusion is carried around the body through the blood flow and attacks healthy cells in nearby organs. This adverse effect of the chemo is what causes the side effects of the treatment, sometimes inflicting chronic crippling pain and illness on the patient.
The World Ovarian Cancer Coalition estimates that by 2035, the number of ovarian cancer cases will increase to 371,000 a year (55%) and deaths will rise to 67% (254,000). Additionally, ovarian cancer, out of all gynecological cancers (which include cervical and endometrial cancers) around the world has a poor survival rate, emphasizing the need for continuous and collaborative research across borders to improve the care outcome for someone with a cancer diagnosis. Furthermore, while it will be years before the actual data is realized, the impact of the global pandemic on patients with a cancer diagnosis has been very significant. One study found that there has been, “substantial decreases in cancer screening, cancer management visits, and cancer surgeries” recently given treatment and care delays due to lockdowns and restricted movements.
Now more than ever obtaining regulatory approval in overseas markets and widening treatment options that do not come with an extensive list of severe side effects is crucial to eradicating cancer across the globe. Led by Ayana CEO Gal Cohen who joined in 2020, it took over a decade to gain FDA approval after launching production efforts of Doxorubicin-HCI in Israel and meeting other FDA regulatory requirements for market authorization in the US. Through a strategic partnership with Padagis who has expertise in bringing specialty pharmaceuticals to the US and Israel, the companies are preparing for commercial launch of Ayana’s Doxorubicin-HCI Liposomal Injection.
CSOFT Health Sciences has written extensively on our continuous support of research and development with cancer treatment and advancements. As part of Breast Cancer Awareness Month, a group of Boston-based CSOFTers participated in The American Cancer Society’s Boston Making Strides of Breast Cancer event which involved walking a 5-mile route around the Charles River and through the streets of downtown Boston to raise awareness and support for the American Cancer society’s extensive research and services offers to cancer patients, survivors, thrivers, and caregivers each year. From medical translations, including women’s health translations and oncology translations, to regulatory consulting, CSOFT Health Sciences, leaders in medical translations, provides end-to-end medical translation services for all phases of the product lifecycle. Learn more about CSOFT Health Sciences’ work with delivering solutions across borders at lifesciences.csoftintl.com!