Health
CSOFT’s health blog aims to provide insight into complex diseases, treatment options and regulatory developments, and other prevalent health issues people face today.
HPV Vaccinations: UK Cervical Cancer Program Shows Success
The results of an England-based national program to vaccinate females against human papillomavirus (HPV) to protect against cervical cancer has shown significant success, new data from England shows. Among this success is a reported 87% decrease in cervical cancer...

FDA Grants Memgen IND Application Clearance
The US FDA (Food and Drug Administration) has granted IND (investigational new drug) application clearance for Memgen’s MEM-288, their cancer immunotherapy treatment for patients with multiple solid tumors. Following this announcement, Memgen, a clinical-stage...

Eli Lilly Retracts From EU, While US Buys Antibody Doses
While Eli Lilly has withdrawn its request for European Union (EU) approval of its COVID-19 antibody-based treatment as the EU pivots its focus on other suppliers, the US has agreed to buy 614,000 additional doses of Lilly's antibody combo for $1.29 billion. The...

Positive Data for Aquestive Therapeutics Sublingual Film
Aquestive Therapeutics, (previously MonoSolRX), known for specializing in the development and commercialization of products that meet unmet needs for patients, has announced positive topline data from its first-in-human Phase 1 PK study of AQST-109 sublingual film for...

Breast Implants: FDA Issues Stronger Risk Warning Labels
The US FDA (Food and Drug Administration) has announced new labels for boxed warnings, to further warn and inform consumer decision-making when considering the risks around breast implants. Included in this announcement, the FDA will also be limiting the sale and...

Daytime Sleepiness (EDS): EMA Approves Bioprojet Ozawade
Bioproject has received approval from the European Medicines Agency (EMA) for their Ozawade (pitolisant) to treat adult excessive daytime sleepiness (EDS) associated with obstructive sleep apnoea (OSA). The approval is based on two Phase III clinical studies to study...

Novavax Files for UK Authorization for its COVID-19 Vaccine
Novavax, a biotechnology company committed to development and bringing to market next-generation vaccines for critical infectious diseases, announced that it has finalized its ongoing regulatory submission to the U.K. Medicines and Healthcare products Regulatory...

Solasia & Nippon Kayaku File for Darinaparsin License Agreement
Solasia Pharma K.K (Salasia) and Nippon Kayaku (Nippon) have announced their joint license agreement for marketing rights for darinaparsin (SP-02) in Japan. Drug candidate developed by Salasia, darinaparsin, aims to treat relapsed or refractory peripheral T-cell...

Resilia Solace Eczema Cream Granted US Commercialization
Resilia Pharmaceuticals has been granted the rights to manufacture, commercialize and sell Solace™ Eczema Cream, a medical device, as an OTC (over the counter) product in the United States. Signing the agreement with Pelle Ventures, the deal will make the cream more...
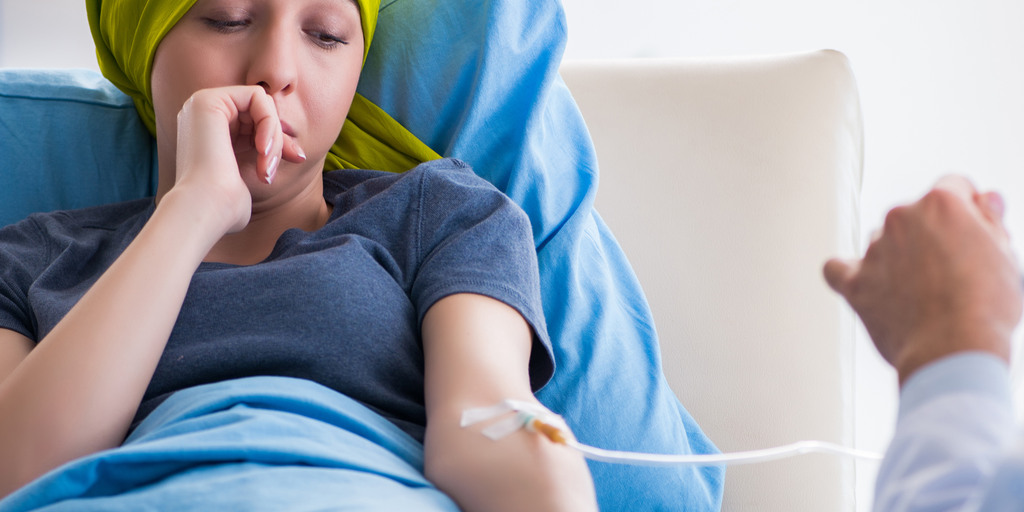
Chemo Side Effects: A Global Challenge for Cancer Treatments
Advancements in global medicine and specialist care are essential to improving quality of life, treatment options, and survival rates for those with a cancer diagnosis, as well as mitigating the side effects that come with receiving those treatments. Recently, the US...
Categories
ABOUT US
CSOFT Health Sciences has over 15 years of experience providing end-to-end translation and localization solutions for all stages of the product life cycle, from pre-clinical to post-launch. We also specialize in China market access consulting services, Asian regulatory and eCTD submissions. Our operation is compliant with ISO 17100 and certified by BSI in ISO 9001:2015 and ISO 13485:2016, providing customized solutions to meet the rigorous regulatory requirements in global submission. For more information, please visit: www.csoftintl.com