An End to Hasty Scribbles: Language AI Puts a Dent in the Tedium of Health Care
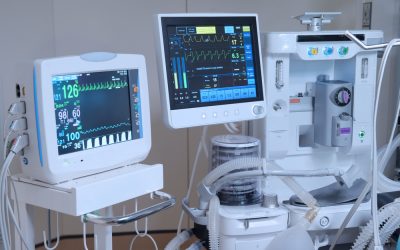
As large language models accelerate clinical research and assist with tasks such as medical writing, language AI is also rapidly appearing in the day-to-day administration of health care, alleviating strains on facilities that can quickly shift from heavy to dire in...
Watch a Video Recap of CSOFT Health Sciences’ 2023 Summer Soiree!
On June 27, CSOFT Health Sciences held its 2023 Summer Soirée at the Harvard Art Museums in Cambridge, MA. Featured as an exclusive accompaniment to our more general presence at this year’s DIA Annual Global Meeting, which occurred simultaneously in Boston’s...
CSOFT Health Sciences Dreams of the Future in a New Trailer for the 2023 Summer Soiree
Last week we shared a brief teaser for the CSOFT Summer Soiree and its centerpiece game, The Shape of Things to Come, where we’ll be asking our guests to submit a prediction about what will occur in the next six years, be it for the Health Sciences or another...
Watch CSOFT’s Teaser Video for Our Summer Soiree!
CSOFT Health Sciences will hold its 2023 Summer Soiree at the Harvard Art Museums in just a few short weeks. We’ve chosen this specific location to bring the worlds of art, communication, and science together in one venue so that we may, like those gathered at DIA...
CSOFT Health Sciences Heads to DIA 2023, Illuminating Opportunities to Drive Diversity in Clinical Trials
As summer arrives in New England, we are excited to announce CSOFT Health Sciences will be returning as exhibitors to the DIA 2023 Global Annual Meeting in our home city of Boston, Massachusetts this June 25th-29th! With our extended team in town for the occasion and...
Multilingual Patient Communications: Parting Lessons of the Pandemic
As the World Health Organization joins the United States in calling an end to the pandemic, few want more than to move on from a time of unrelenting challenges, but that timing may not be so fortuitous when it comes to capturing its most important lessons for...
MedTech and Medical Device Localization: Leveraging ISO 13485 for Multilingual Quality Management
With so much of MedTech resembling a medical device, and with more medical devices incorporating software and other digital components, it is ever harder to define what is a MedTech and what is a medical device localization project. With the possibility some projects...
Linguistic Validation for eClinical Patient Questionnaires: Driving Patient Centricity Through eCOA Translations
When it comes to setting up a foundation for diverse, patient-centric clinical trials, linguistic validation is an essential localization practice that can help prepare patient questionnaires for participants in diverse global regions, an essential to succeeding in...
Reducing Clinical Trial Delays with IVD Translations
When conducting clinical trials in Europe and around the world, accurate and effective IVD translations help mitigate clinical trial delays that stand in the way of device approval. In vitro diagnostic medical devices (IVDs), also called in vitro diagnostic products,...
Can Clinical Trial Translations Improve Sponsor Diversity Index?
Following the premiere of Bioethics International’s new diversity index in 2023, how can clinical trial translations improve a company’s future rating? Diversity in clinical trials has been a hot topic in recent years, even prompting the U.S. Food and Drug...
Personalization and Patient-Centricity in Pharmaceutical Labeling Translations
Among the many steps it takes to bring a drug to market, pharmaceutical labeling translations may seem like one small hurdle to overcome on the way to approval. Medical translation of pharmaceutical labels, which can include labels on the carton or package and a host...
ChatGPT in Healthcare: A Tool to Improve Patient Communication and Medical Translations?
Since OpenAI publicly released ChatGPT, a generative artificial intelligence (AI) that uses a large language model to produce human-like text, the possibilities for ChatGPT have taken just about every communication and translation-heavy industry by storm. But in the...
ePRO Translations: Cultural Adaptation for the Decentralized Clinical Trial
Patient-reported outcome (PRO) translations have long required linguistic validation before use in clinical trials, so with the rise of digital instruments in decentralized clinical trials, what additional considerations are there for electronic PRO (ePRO)...
eLearning Translations: Improving Medical Device Use Around the Globe
How can medical device companies leverage eLearning translations to improve the use of medical devices around the world? While patients are the end user for many medical devices, healthcare professionals are responsible for guiding patients in correct use, as well as...
Clinical Trial Corner: Quantifying the Socioeconomic Value of Industry Clinical Trials for Participating Countries
Clinical Trial Corner is a biweekly series from Dr. Vladimir Misik, founder of LongTaal and CSOFT’s VP of Global Clinical Strategy. In our previous CT Corner posts we assessed patient diversity on a global scale and flagged countries and regions that appear to be...
Patient Recruitment Translations: A Treatment for the Reputation Disparity in Clinical Trials?
How can patient-facing materials and patient recruitment translations facilitate recruitment of patients particularly to countries underrepresented in global drug development? As we discussed in our latest Clinical Trial Corner post, some countries and regions,...
Clinical Trial Corner: Assessment of Countries’ Reputation Among Sponsors of Industry Clinical Trials
Clinical Trial Corner is a biweekly series from Dr. Vladimir Misik, founder of LongTaal and CSOFT’s VP of Global Clinical Strategy. In the previous two-part Clinical Trial Corner miniseries, we provided a global assessment of patient diversity in industry CTs (iCTs)...
Neurocrine Biosciences Presents Additional Phase 3 Data for KINECT-HD Study Evaluating Valbenazine for Chorea Associated with Huntington Disease at HSG 2022
Neurocrine Biosciences, Inc. (NASDAQ: NBIX), a leading neuroscience-focused biopharmaceutical company, reported additional results from the Phase 3 KINECT-HD study investigating valbenazine for the treatment of chorea associated with Huntington Disease (HD). In...
AbCellera’s First Program with Regeneron in Multi-Target Collaboration Advances in Preclinical Development
AbCellera (Nasdaq: ABCL) announced that Regeneron has elected to exercise its right to advance a therapeutic antibody candidate, discovered in partnership with AbCellera as part of a multi-target collaboration between the companies, into further preclinical...
Unity Bio Set to Launch Pivotal DME Study after Significant Data
Shares of Unity Biotechnology surged in early trading Tuesday following the company’s release of Phase II data showing a single injection of its experimental drug provided visual improvements in patients with diabetic macular edema. A single injection of UBX1325 led...