What are Clinician-Reported Outcomes (ClinROs), and why are their translations so important for companies conducting clinical trials? ClinROs are valuable assessment reports written by a clinician or trained health care professional and are used to document measurable changes in a participant’s overall health, symptoms, quality of life, and ability to function during a clinical trial. With more clinical trials being conducted overseas and the importance of accurately documenting clinical outcomes across languages, high-quality and accurate clinician-reported outcomes translations are vital to meeting the needs of all stakeholders in the clinical trial process.
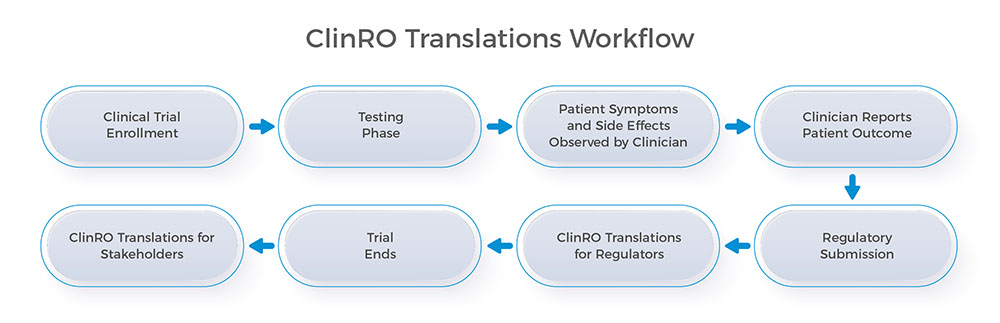
To help clinicians and medical professionals accurately document changes to a patient’s health across any language and meet international language requirements for clinical trial documents, CSOFT Health Sciences provides high-quality and cost-effective ClinRO translations in over 250 languages. With a global network of 10,000+ in-country linguists and subject matter experts, we provide a range of COA translations, including patient-reported outcome (PRO) translations, performance-reported outcome (PerfO) translations, and observer-reported outcome (ObsRO) translations.

Case Report Form (CRF) Translation Services
During a clinical trial, case report forms (CRF/eCRF) are an essential tool to record and collect data on each trial participant. This makesCRF translations necessary for sponsors conducting trials across borders to reach patients in multiple languages. To help the sponsor support and test their hypothesis, a CRF is created and used to gather vital patient information, which can range from a small collection of notes to high volumes of clinical notes and patient data. With a growing demand to reach and engage patients across languages and to help sponsors comply with Institutional Review Board (IRB) guidelines, CSOFT Health Sciences provides professional and high-quality CRF translations, ensuring that the already-complex trial process isn’t complicated by miscommunication.
Learn more about our case report form (CRF) translation services.
Linguistic Validation and Harmonization
Our ClinRO translation services include a comprehensive linguistic validation and harmonization process to ensure that the translated content maintains the intended meaning and clinical significance of the original outcomes. This process involves forward and backward translations, expert reviews, and cultural adaptation to ensure accurate interpretation across multiple languages.
Learn more about our linguistic validation for COAs.

Streamlining Global Reach with Clinical Study Report Translations
With the expansion of new medical treatments worldwide, the need for clinical study report translations grows to facilitate the analysis of study methods and results. As organizations seek to introduce novel treatments to international markets, adherence to global regulatory standards is paramount to ensure trustworthiness. CSOFT Health Sciences leverages a global team of in-country linguists and subject matter experts to deliver high-quality, tailored medical translation and localization solutions for clinical study reports.
Learn more about our clinical study report translations.
Quality Assurance
CSOFT Health Sciences has developed a process for quality assurance to ensure that every medical translation project meets quality standards in a cost-effective and timely manner. We are certified in ISO 17100:2015, ISO 9001:2015, and ISO 13485:2016 to ensure our customized solutions meet global regulatory requirements. Our subject matter expert linguists have at least seven years of experience and work with in-country reviewers and project style guides to meet industry standards. CSOFT offers an online translation management ecosystem for one central location to leverage real-time translation memory and terminology management through our innovative cloud-based technology. Every step of the way, CSOFT has you covered.
Learn more about our quality assurance process.
Data Security
With over 20 years of experience in medical translation, CSOFT Health Sciences understands the importance of data security to our clients, and we take nothing for granted when confidentiality is a concern. Our well-documented and fully traceable information data security policies, checklists, and quality records leverage the best practices of ISO 27001. They are designed to protect everything from source data to translations. From our 24/7/365 data monitoring and advanced encryption to our access control measures, you can be sure that your project data is safe from start to finish.
Learn more about how CSOFT prioritizes data security.

