Health
CSOFT’s health blog aims to provide insight into complex diseases, treatment options and regulatory developments, and other prevalent health issues people face today.
Happy International Women’s Day
CSOFT joins the Health Sciences industry in recognizing International Women's Day, Women’s History Month and the important contributions women make to society. The FDA recently released the 2019 Drug Trials Snapshots Report. This report summarizes the demographic...

Communicating Public Health to Multilingual Populations: The Example of Singapore
With the ongoing global COVID-19 outbreak, public health policy is being put to the test. To educate people and modulate their health, sanitary, and hygienic behavior, it’s crucial that policymakers communicate effectively. Public health guides inform and protect...

China Creating a Framework on Real-World Evidence
Purpose of Real-World Evidence/Real-World Data Real-World Evidence is critical for designing more effective treatments. For example, to test new treatments, pharmaceutical companies adopt a method called "Randomized Controlled Trial" (RCT). There are two design groups...

A Step Forward in Personalized Care: Translational Research and Precision Medicine
Our healthcare system, including translational research and precision medicine, has come a long way over the past decade. Targeted vaccine breakthroughs and ongoing improvements in cancer treatment have transformed our ability to survive and recover from health issues...

Assisted Reproductive Technology: Creating Life with ART
ART and Society For some, having children is an ultimate goal in their own life-progression. For those unable to become pregnant naturally, Assisted Reproductive Technology (ART) can bring hopeful parents the successful pregnancy they desire. ART takes many forms,...
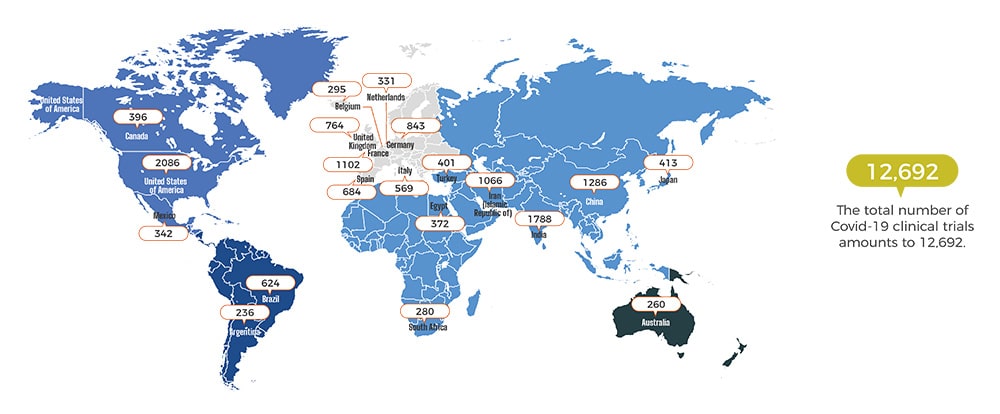
Covid Spreading at an Alarming Rate
Where it is originated from? In December 2019, Covid appeared in a seafood market located in Wuhan, the capital of Central China’s Hubei province. Researchers traced back to the first patient and he did not come into contact with the seafood market. Up until now, the...

A Look Back: New York’s Learning-by-Doing Response to a Pandemic
With thoughts of the latest pandemic on so many of our minds, it can be useful to look to history for context and reassurance as we try to understand our current predicament. Often, it is easy to become lost in a vague picture of the developing world as the sole...
Covid: Calling All Heroes
Language’s fix to a world problem In dedication to Dr. Li Wenliang As a sudden outbreak with untold global health implications, the 2019 Coronavirus exemplifies a crisis scenario where the quality and character of the immediate response has the power to shape future...

China Finalized the Trade Agreement for Patent Drug with the U.S.
Trade Agreement On January 16, 2020 the U.S. and China finalized the trade agreement that specifies the patent protection rules for drugs. Under the patent protection, biopharmaceutical companies can fill out an application and the term of each new patent is 20 years....

Tracking the Coronavirus With AI: How JHU & BlueDot are Leading Pre-vaccine Outbreak Response
To understand and control the spread of the latest coronavirus (2019-nCOV), a vast array of artificial intelligence and natural language processing tools have been deployed, giving those watching from the Health Sciences field good cause to be optimistic about recent...
Categories
ABOUT US
CSOFT Health Sciences has over 15 years of experience providing end-to-end translation and localization solutions for all stages of the product life cycle, from pre-clinical to post-launch. We also specialize in China market access consulting services, Asian regulatory and eCTD submissions. Our operation is compliant with ISO 17100 and certified by BSI in ISO 9001:2015 and ISO 13485:2016, providing customized solutions to meet the rigorous regulatory requirements in global submission. For more information, please visit: www.csoftintl.com