Health
CSOFT’s health blog aims to provide insight into complex diseases, treatment options and regulatory developments, and other prevalent health issues people face today.
Moderna Notes Early Signs of Success in Covid Vaccine
Today (5/18) Moderna announced that its Covid vaccine clinical trial in the United States has started to see protective antibodies in data from 8 healthy individuals, chosen at random from a 45-person study. The study, which began in March, and found that the...

Potential for Covid Vaccine to be Approved Within a Year, According to EMA Official
Marco Cavaleri, the European Medicines Agency’s (EMA) top official overseeing anti-infectives and vaccines, acknowledged the potential for a Covid vaccine to be ready by Spring 2021 during a virtual conference last Thursday (5/8). He says that while there may be...

FDA Approves Eli Lilly’s Retevmo, the First Treatment for Patients with a Certain Genetic Mutation for Lung and Thyroid Cancers
The FDA announced on Friday (5/8) of its approval for Eli Lily's Retevmo, a drug which treats three kinds of tumors associated with lung and thyroid cancer: non-small cell lung cancer, medullary thyroid cancer and other types of thyroid cancers. This comes as the...

Clinical Trials: How Real-World Data and Real-World Evidence Are Revealing the Patient’s Untold Story
As our world is constantly changing, the healthcare industry, especially clinical trials, are adapting to better serve patients and improve lives around the world. While healthcare is shifting to become more patient-centric, additional data from the patient’s...

Medical Writing: Authoring a Clinical Study Protocol – I
Continuing her interview series, Dr. Nimita Limaye further answers questions from our guest audience members surrounding medical writing practices for clinical study protocols. She focuses on the role a medical writer plays in the process of writing these strategic...

Moderna Moves into Phase 2 of Covid Vaccine
In their business update, Moderna announced they received FDA clearance to move into Phase 2 of their study for the COVID-19 vaccine. In their press release, they also reported additional funding from Biomedical Advanced Research and Development Authority (BARDA) as...
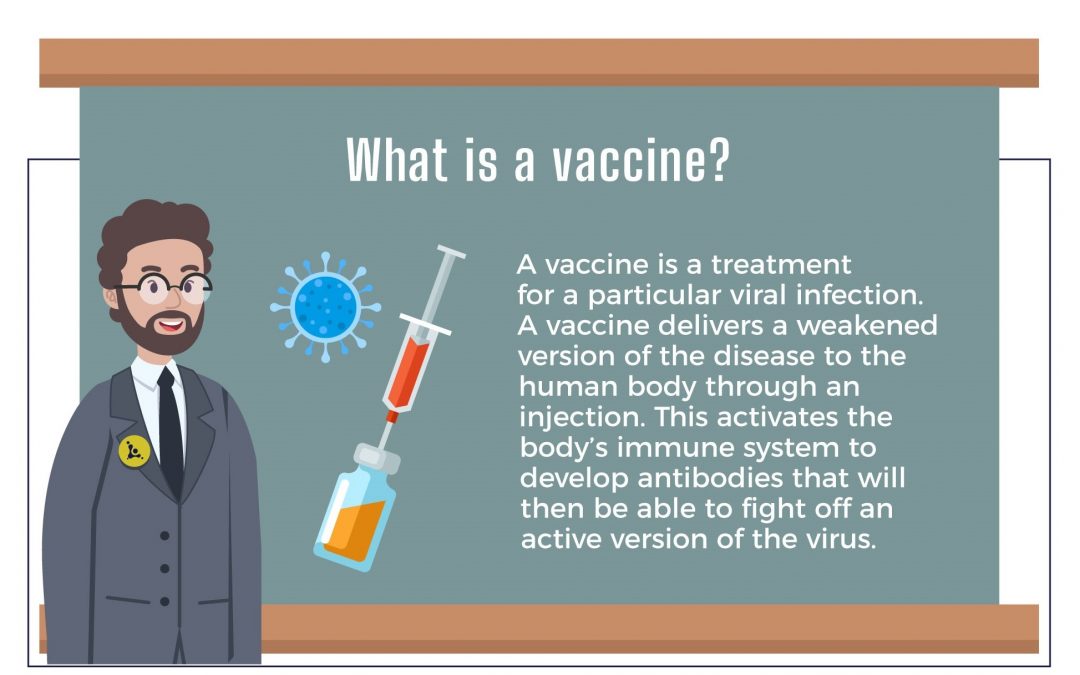
Exploring the World of Vaccinations
Explore the world of vaccinations with Professor K in understanding what they are, how they work, and the challenges they face in development and implementation, especially for COVID-19.

Key Factors Driving Medical Writing Outsourcing Strategy – I
CSOFT Health Sciences SVP Dr. Nimita Limaye has taken the time to answer some questions from our guest audience about the medical writing industry. In her first interview, she focuses on key factors that cause businesses to outsource their medical writing needs. Jane:...

FDA Amends Policy for Covid Tests
The FDA announced that it would strengthen its oversight for companies marketing the Covid serology tests. After questions came to light in regards to the accuracy of some of the testing kits, the FDA is now requiring commercial manufacturers to submit an emergency...

FDA Grants EUA for Covid Treatment Remdesivir from Gilead
The FDA announced today (5/1) that it would grant EUA for Gilead for their antiviral drug remdesivir for treatment in early response to suspected or confirmed patients with Covid. This authorization will allow the distribution of the drug all over the US, as well as...
Categories
ABOUT US
CSOFT Health Sciences has over 15 years of experience providing end-to-end translation and localization solutions for all stages of the product life cycle, from pre-clinical to post-launch. We also specialize in China market access consulting services, Asian regulatory and eCTD submissions. Our operation is compliant with ISO 17100 and certified by BSI in ISO 9001:2015 and ISO 13485:2016, providing customized solutions to meet the rigorous regulatory requirements in global submission. For more information, please visit: www.csoftintl.com