Clinical Trial Corner is a biweekly series from Dr. Vladimir Misik, founder of LongTaal and CSOFT’s VP of Global Clinical Strategy.
In the first part of my blog of this two part series on patient diversity in industry CTs (iCTs), I summarized the importance of the recent US FDA guidance to the industry demanding enrollment of representative numbers of participants from underrepresented racial and ethnic populations in the United States (1) and pointed out that regulators in several other countries should be equally concerned about proportionality of their patients’ representation in global iCTs. To that end I presented data demonstrating gaps on a global scale in patients’ access to iCTs. (2)
In this blog, as promised, I will shed more light on the topic of proportionality of global patient access to iCTs. Beyond comparing patient accessibility to iCTs merely on a per capita basis as we have done in the previous blog, here I will focus on highlighting potentially more concerning global imbalances: imbalances between participation of patients in several ethnically and culturally distinct global geographies in the consumption of developed pharmaceuticals, relative to their participation in development of novel biopharmaceutical products.
To assess these imbalances we have utilized our proprietary methodology LongTaal Clinical Trials Landscape dashboard: for each country, participation of patients in consumption of developed pharmaceuticals was expressed as global market share of pharmaceutical sales of prescription pharmaceuticals, while participation in development of novel biopharmaceutical products was expressed as a global share of active iCTs sites (iCT market share). Regarding additional details about the methodology, let me refer you to our recent publication where these terms and the data sources and methodology we utilized in obtaining them are explained. (3)
For this purpose, we introduced a quantifiable measure of balance between participation in development of novel pharmaceuticals relative to consumption of marketed products, a so-called Country Biopharma Products Consumption vs Development Participation index (BP-C/D index), with Consumption approximated as % of global sales in that country and Participation approximated as % of global iCT sites:
BP-C/D index = Share of prescription sales (% of global sales) / Industry CT market share (% of global iCT sites)
We consider a “normal” or acceptable range of BP-C/D index = <0.5 – 5>, and would flag anything > 10 as potentially significantly out of balance: i.e. patients in the impacted countries are potentially consuming medications in development of which patients with similar ethnic or cultural profiles have not been adequately represented.
We recognize the following limitations of such a top-level approach:
- Significant pricing differences between countries, i.e., typically lower pricing for the same compound in lower income countries
- Analysis is not done at the level of the product and/or therapeutic area
These limitations notwithstanding, it can serve as a helpful identifier of significant imbalances. It is safe to assume that countries where the BP-C/D index is near or exceeds 10 are under-represented in the development of novel pharmaceuticals, and thus potentially consuming medications in development of which patients with similar ethnic or cultural profiles have not been adequately represented. (For how many of the drugs approved across countries where Ramadan is observed has the impact of fasting on metabolism and/or on their safety and efficacy of these drugs been studied?)
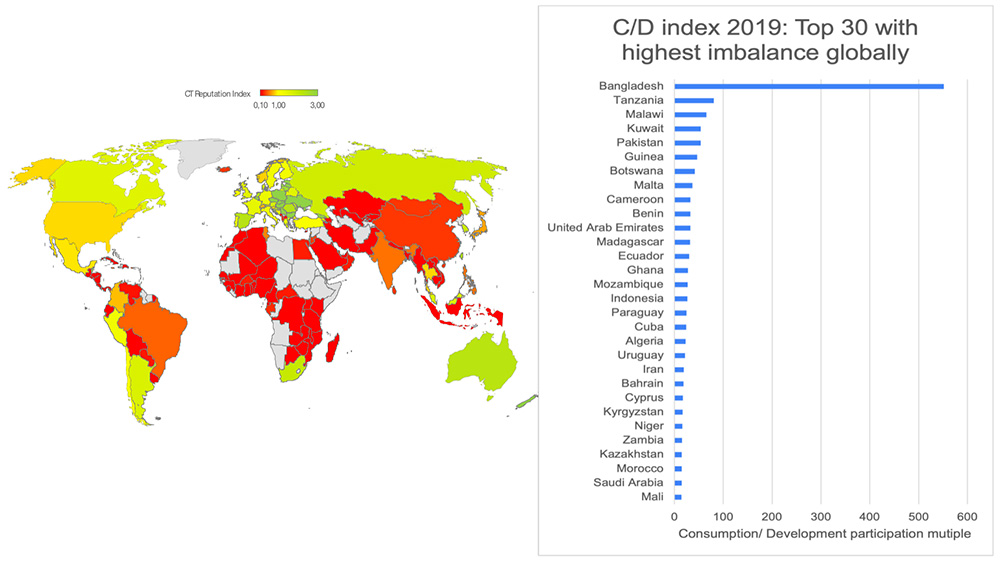
Graphical presentation of the results in Figure 1 shows the global heat map of BP-C/D index by country around the globe (with darker tones of red showing the problem “out of balance” areas). The graph insert shows the top 30 list of countries with the highest imbalances between participation in consumption of marketed products relative to participation in development of new products (highest BP-C/D multiple), with a was majority of the impacted countries from Africa and the Middle East.
Note: Of interest to the reader may be that the inverse function (i.e. iCT market share/share of prescription sales) can be used to assess reputation of countries among sponsors of iCTs. (3) I plan to shed more light on countries’ reputation among sponsors of iCTs in one of my upcoming blogs.
Figure 1. Biopharma Consumption/ Development Index. Data source: LongTaal Clinical Trials Landscape dashboard, 2019 data.
In summary, here we have illustrated another dimension of patient diversity gaps in iCTs: patients from countries/ regions around the world which appear to be under-represented in development of novel pharmaceutical relative to consumption of marketed products, and thus potentially consuming medications in development of which patients with similar ethnic or cultural profile have not been adequately represented. The most impacted global regions are Africa and the Middle East with the most countries with the highest global imbalances. In these regions as well as other countries with significantly skewed BP-C/D balances, this problem should be further analyzed at a product level and based on the findings of a robust discussion involving country regulators, health policy experts, insurance providers, and biopharma representatives about what proposed remedies should take place. Possible options include for example requiring a sub-study on a representative population of ethnically identical or similar patient groups as part of the marketing authorization, and/or mandatory real world post launch data collection allowing assessment of product safety and efficacy in local populations.
We believe that in the wake of the recent US FDA guidance flagging the lack of adequate ethnic diversity in iCTs, there is an opportunity to initiate discussions around this problem in the affected geographies. It remains to be seen whether the market significance of these markets is such that biopharma is prepared to play a more active role.
About Dr. Misik
Dr. Misik has 30+ years’ experience of working in biomedical R&D at the George Washington University, School of Medicine and at the National Cancer Institute in Bethesda, Maryland. In addition to holding several executive roles in multinational CROs, Dr. Misik founded LongTaal, a company that maintains up-to-date dashboards and visualizations of the global CT scene by introducing a range of tools, including a CT patient accessibility index and country CT reputation index.
REFERENCES
- Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry. Draft Guidance. US FDA, Rockwille, MD, USA. April 2022. Access here.
- Misik V: Patient Diversity In Clinical Trials: Why It Matters, Who Should Be Concerned, and What Can Be Done. CSOFT Health Sciences, Clinical Trial Corner Blog, Sep 22, 2022. Access here.
- Misik V, Jarosz B, Beckowski L, Czarnecka M, Dabrowski T, Drake D, Milowska K, Ziecik P, Magielski P, Szczepannik W: REPORT: Industry Clinical Trials in Poland. Possibilities to increase number and scope of trials in Poland. Publishers: INFARMA and POLCRO. December 2021; pp 1-115. Access here.