Clinical Trial Corner is a biweekly series from Dr. Vladimir Misik, founder of LongTaal and CSOFT’s VP of Global Clinical Strategy.
The US FDA is rightfully concerned about patient diversity in industry-sponsored clinical trials (iCTs). (1) In its recent guidance to the industry, the FDA states: The purpose of this guidance is to provide recommendations to sponsors developing medical products on the approach for developing Race and Ethnicity Diversity Plan to enroll representative numbers of participants from underrepresented racial and ethnic populations in the United States, such as Black or African American, Hispanic/Latino, Indigenous and Native American, Asian, Native Hawaiian and Other Pacific Islanders, and other persons of color, in clinical trials. Individuals from these populations are frequently underrepresented in biomedical research despite having a disproportionate disease burden for certain diseases relative to their proportional representation in the general population.
Therefore, sponsors should develop operational measures that can be implemented to ensure diverse clinical trial participation and to improve the generation of evidence regarding safety and effectiveness across the entire population. The FDA continues:
Such measures could include but are not limited to offering financial reimbursement for expenses incurred by participation in a clinical trial or study (e.g., travel or lodging), providing language access to participants with limited English language proficiency, and partnering with community-based organizations to provide support to study or trial participants.
The racial and ethnic underrepresentation in today’s iCTs in the US is on track to be amplified further in coming years, as the US is set to become “minority (non-Hispanic) White” by 2045. (2)
The problem of insufficient representation of certain ethnic or racial groups in iCTs is not new and certainly should not be a concern to the US regulators only and as mentioned below there are nations/ethnicities with insufficient representation in development of new drugs around the world.
How did we get here? The root causes are multiple. First and foremost, we need to acknowledge the contribution of deplorable historical practices, where ethnic minorities and/or vulnerable populations were involved in research we now consider unethical. (3) (4)
The impact of this legacy continues to affect the inclusion of CT subjects today in two significant ways:
- Mistrust in the healthcare system among ethnic minorities, thus lowering their willingness to participate in CTs offered to them. (5)
- Reverse selection bias among healthcare professionals, who are sensitized about potential ethical risks. Well-meaning health professionals may limit subjects’ participation in a study involving vulnerable populations under the guise of protecting these individuals from harm. (6)
Other factors may be at play as well, and their collective manifestation is the underrepresentation of certain socioeconomic or ethnic groups in clinical trials. In their 2013 paper, Noor et al. studied access to early-phase cancer trials in the UK as a factor of the socioeconomic statuses of patients, finding that the least deprived patients were almost twice as likely to be referred for an early-phase oncology clinical trial. Ethnicity analysis demonstrated that the non-white population was less likely to be recruited. (7) In another study, Godden et al. analyzed the effect of the ethnicity of patients recruited to cancer clinical trials of all phases in one hospital trust in England, finding that non-white minorities were 30% less likely to be recruited than were white patients. (8)
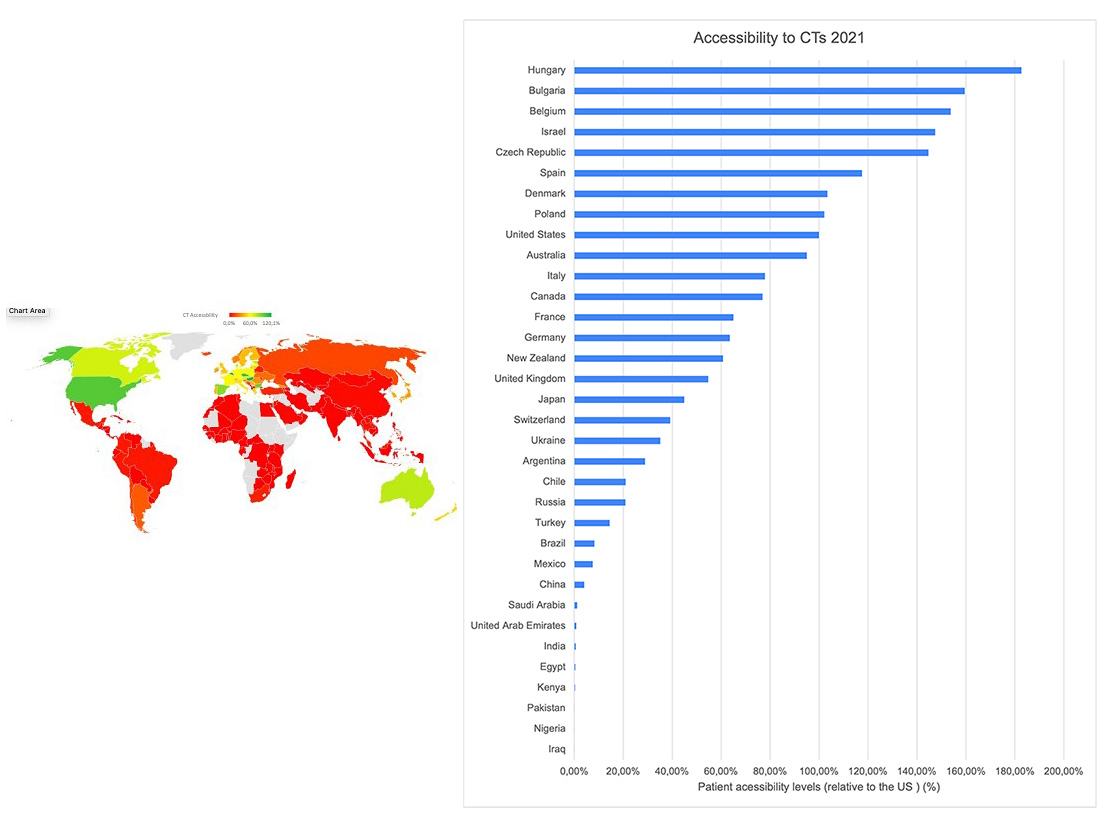
In addition, and as Figure 1 demonstrates, gaps in the access to and participation in iCTs also exist on a global scale, as accessibility to iCTs for patients in several ethnically and culturally distinct global geographies lags substantially behind the countries in North America and Europe. (9) (10)
Figure 1. Accessibility to clinical industry clinical trials, calculated as number of clinical trial sites per 1m population relative to the US levels (US = 100%). Data source: LongTaal Clinical Trials Landscape dashboard/Clinical Trials Indices (www.longtaal.com)
I plan to shed more light on this topic in one of my upcoming blogs, in which I will demonstrate that beyond the aforementioned geographic gaps in accessibility to iCTs, significant geographic imbalances between participation in the development of new products and participation in consumption of developed pharmaceuticals exist, and the ramifications of these.
Why is this a problem? As just a few examples below will illustrate, racial, ethnic, and cultural factors impact the safety and efficacy profile of drugs and medical devices. Such factors must be therefore taken into consideration both during a products’ development stage and after their launch, including:
- Skin pigmentation: Pulse oximeters function less accurately in patients with higher levels of skin pigmentation (darker skin), resulting in a risk of missing clinically important hypoxia. (11) This is possibly one of the contributing factors that led to the disproportionally high COVID-19 related death rates among Black or African American, as well as Hispanic or Latino populations in the US. (12)
- The effects of race and ethnicity on drug metabolism: Significant racial and ethnic variations in the pharmacokinetics, efficacy, and toxicity of drugs have been reported. (13) (14)
- Religious and cultural practices: Drug metabolism rates could also be influenced significantly by environmental and nutritional factors such as fasting (e.g. during Ramadan). The resulting changes in drug metabolism may result in treatment failure or, conversely, in increased side effects or toxicity. Studies have shown that fasting alters drug metabolism by modulating the activity of the drug metabolizing enzymes involved. (15)
Let me touch here on two additional factors that, while not immediately obvious, may be either be part of the solution or be part of the problem:
- The role of technology:
- Decentralized or virtual CT solutions have been finally embraced by the industry during the COVID-19 pandemic and supported by processes and technologies enabling telemedicine and remote patient visits. Some leaders in innovative technology-based solutions enabling and supporting DCTs are Science 37, Medable, and Lightship. While such technologies hold a lot of promise, the practical impact on improving patient diversity in CTs is yet to be determined.
- EHR mining with technology-based EHR-mining solutions for clinical trials, such as those offered by TriNetX or Clinerion, appear to be uniquely suited for the identification of trial sites with access to diverse patient populations.
- Patient expense reimbursement: The US FDA in its guidance to the industry specifically called out offering financial reimbursement for expenses incurred by participation in a clinical trial or study (e.g., travel or lodging) as a tool to improve patient diversity. (1) If handled well, this indeed can be an effective tool. However, as a recent article points out, there is an acute lack of uniform approach, including among regulators, globally. (16) As a result of these differences, some geographies and regulatory jurisdictions offer no reimbursement of (often very significant) travel expenses, and/or no compensation of lost wages; alternately, the process of reimbursement of these expenses may be ineffective or slow. This naturally impacts low earners disproportionally, thus reducing their interest to participate in a CT or increasing their trial drop-out rates, both adversely impacting patient diversity in iCTs.
In summary, patient diversity in iCTs is a real problem that the US FDA recently called out, and industry started paying attention. However, insufficient patient representation of certain ethnicities should by no means concern US regulators alone; patient diversity should also be a concern of regulators and healthcare professionals in countries that are not adequately represented in development on new products. In addition to the solutions called out by US FDA in its draft guidance (e.g. offering financial reimbursement for expenses incurred by participation in a clinical trial or study, providing language access to participants with limited English language proficiency, and partnering with community-based organizations to provide support to study or trial participants), technologies, including technology-enabled virtual clinical trials and EHR data mining, hold a lot of potential for addressing the issue of inadequate patient diversity in clinical trials.
About Dr. Misik
Dr. Misik has 30+ years’ experience of working in biomedical R&D at the George Washington University, School of Medicine and at the National Cancer Institute in Bethesda, Maryland. In addition to holding several executive roles in multinational CROs, Dr. Misik founded LongTaal, a company that maintains up-to-date dashboards and visualizations of the global CT scene by introducing a range of tools, including a CT patient accessibility index and country CT reputation index.
REFERENCES
- Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry. Draft Guidance. US FDA, Rockwille, MD, USA. April 2022
- Frey FH. The US will become ‘minority white’ in 2045, Census projects. Brookings; March 14, 2018. https://www.brookings.edu/blog/the-avenue/2018/03/14/the-us-will-become-minority-white-in-2045-census-projects/.
- Department of Health, Education, and Welfare. The Belmont Report. Ethical Principles and Guidelines for the Protection of Human Subjects of Research. U.S. Department of Health & Human Services 18April1979 https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html#:~:text=Three%20basic%20principles%2C%20among%20those,of%20persons%2C%20beneficence%20and%20justice.
- Centers for Disease Control and Prevention. The U.S. Public Health Service Syphilis Study at Tuskegee. The Tuskegee Timeline. CDC, 22 April 2021 https://www.cdc.gov/tuskegee/timeline.htm.
- Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D; More than Tuskegee: Understanding Mistrust about Research Participation. J Health Care Poor Underserved. 2010 Aug; 21(3): 879–897 https://muse.jhu.edu/article/389044
- Sutton LB, Erlen JA, Glad JM, Siminoff LA; Recruiting vulnerable populations for research: Revisiting the ethical issues, Journal of Professional Nursing, Volume 19, Issue 2, 2003, pp 106-112. https://doi.org/10.1053/jpnu.2003.16
- Noor AM, Sarker D, Vizor S, McLennan B, Hunter S, Suder A, Moller H, Spicer JF, Papa S. Effect of Patient Socioeconomic Status on Access to Early-Phase Cancer Trials. J Clin Oncol 31 (2013) pp. 224-230
- Godden S, Ambler G, Pollock AM. Recruitment of minority ethnic groups into clinical cancer research trials to assess adherence to the principles of the Department of Health Research Governance Framework. J Med Ethics 36 (2010) pp. 358-362
- Misik V, Brady RV, Bolecek M, Klech H. Current Trends in Globalization of Industry-Sponsored Clinical Trials, Applied Clinical Research, Clinical Trials & Regulatory Affairs, 1 (2014) 56-66
- Misik V, Bolecek M, Brady RV. Ethical Considerations of Industry-Sponsored Clinical Trials in the Arab Region. In: Research Ethics in the Arab Region. Silverman H (eds.); Springer 2017, pp 161-170
- Okunlola OE, Lipnick MS, Batchelder PB, Bernstein M, Feiner JR, Bickler PE. Pulse Oximeter Performance, Racial Inequity, and the Work Ahead. Respiratory Care, 67 (2022) 252-257
- The COVID Racial Data Tracker. COVID-19 is affecting Black, Indigenous, Latinx, and other people of color the most. The COVID Tracking Projects at the Atlantic. March 7, 2021. https://covidtracking.com/race.
- Johnson JA. Influence of race or ethnicity on pharmacokinetics of drugs. J Pharm Sci 86 (1997) 1328-33
- Phan VH, Moore MM, McLachlan AJ, Piquette-Miller M, Xu H, Clarke S. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opinion on Drug Metabolism & Toxicology, 5 (2009) 243-257
- Lammers LA, Achterbergh R, Mathot RAA, Romijn JA, The effects of fasting on drug metabolism, Expert Opin Drug Metab Toxicol. 16 (2020) 79-85
- Novak K. Should We Pay Patients in Clinical Trials For Their Time? Clinical Leader, 29 September 2022.