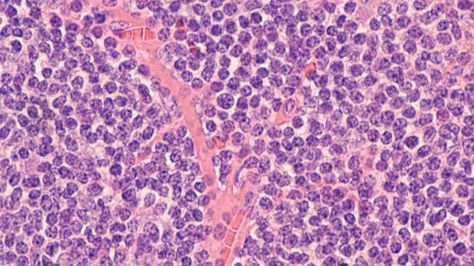
On November 14, 2019, the U.S. Food and Drug Administration gave approval to BeiGene Ltd’s lymphoma treatment, Brukinsa. BeiGene is a Chinese biopharmaceutical company that focused on cancer treatments. The treatment “Brukinsa” has been tested on 118 patients with mantle cell lymphoma. Mantle cell lymphoma is a type of cancer that involves white blood cells growing uncontrollably and turning into “tumors.” These white cells are able to spread to other tissues by entering the lymphatic channels and bloodstream. Mantle cell lymphoma disease mostly occurs in men over the age of 60. After successfully running the trial, the FDA immediately approved the treatment for adults only if they have one previous treatment for the disease before. BeiGene will settle on the price once the treatment is available. The FDA has taken quite an interest in expediting drugs for oncology from China and expecting Beigene to sell drugs globally, including the U.S. market.
Reference
Mathias, T. (2019, November 14). China’s BeiGene gets FDA approval for drug to treat rare form of lymphoma. Retrieved November 20, 2019, from https://www.reuters.com/article/us-beigene-fda/chinas-beigene-gets-fda-approval-for-drug-to-treat-rare-form-of-lymphoma-idUSKBN1XO2UI.
O’Connor, O. A. (2014, November). Mantle Cell Lymphoma Facts. Retrieved November 20, 2019, from https://www.lls.org/sites/default/files/file_assets/mantlecelllymphoma.pdf.