While COVID-19 has caused many interruptions across industries, the drug development and supply chain has experienced some of the most severe repercussions. With potential exposure to the virus limiting number of trial participants, as well as site staff and physicians, clinical trials all over the world have either been interrupted, delayed, or cancelled. While regulatory bodies, like the U.S. Food and Drug Administration (FDA), have issued guidances for conducting clinical trials during the pandemic, there are still many obstacles that CROs and pharmaceutical companies face including regulatory prioritization of other clinical trials, clinical site closures, lack of accessibility for patients due to halted transportation for quarantine measures, and inadequate informational resources to better patient communication. From these issues, however, there is an innovative response to meet the needs of patients worldwide through active communication and engagement, educating and empowering clinical trial participants to take ownership of their health.
According to GlobalData, since COVID-19 cases started to spike all over the world, in particular the U.S., over 100 companies have announced interruptions in clinical trials. With thousands of clinical trials facing issues in delays, as well as an adjustment in research to address the COVID-19 pandemic, it is likely that even after a vaccine and treatments are approved, the after effects will be felt for many years to come. Brooke Wilson who is the Associate Director of Trials Intelligence at GlobalData, comments, “The number of disrupted clinical trials and organizations has continued to grow over the last three months. This trend is most noticeable between April and May, but has begun to slow, as depicted in the figure. Suspension of enrollment accounted for 61.5% of the disruptions. Over all three months, 14% of the disrupted clinical trials are specifically Pivotal/Registrational, giving an indication that there will be an impact on regulatory approvals in the future.”
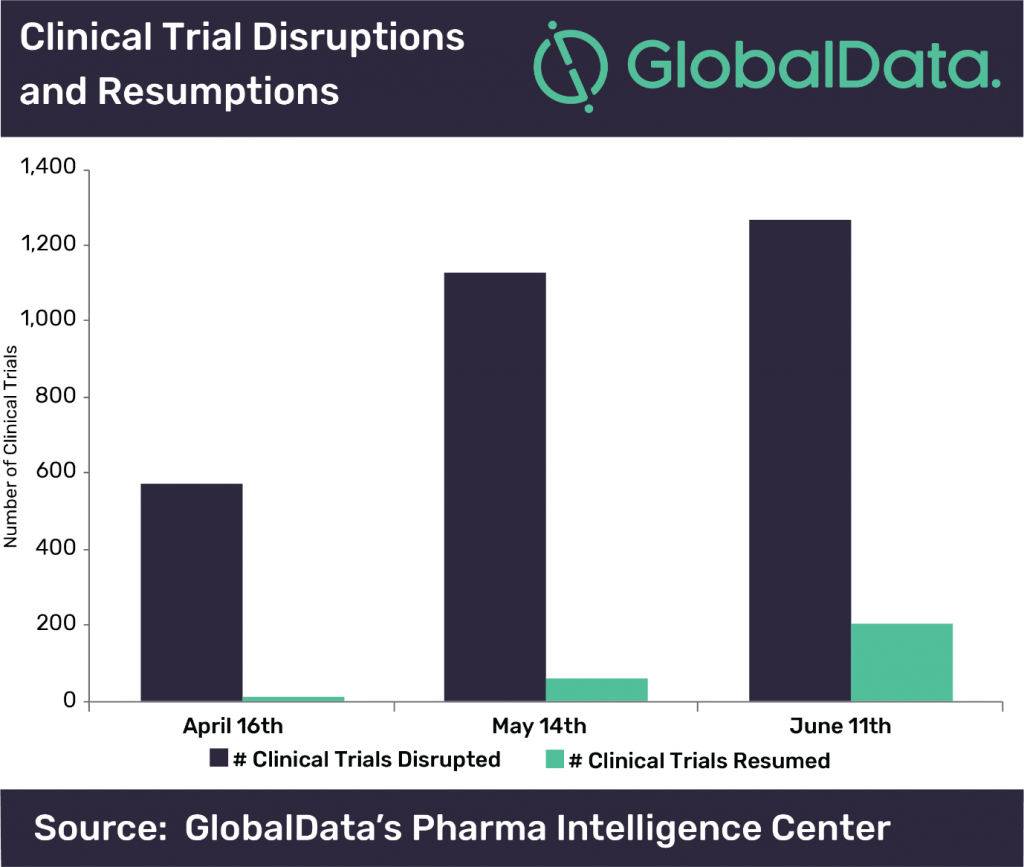
Some countries are working to mitigate the issues for regulatory approval, however. The U.S. FDA issued guidances for those conducting clinical trials during the pandemic on how to best protect patient safety while also continuing good clinical practice and minimizing risks to trial integrity. The European Medicines Agency (EMA) also released their own guidances for life science companies conducting clinical trials in light of COVID-19. Even with these guidances and additional safety measures, however, patients still face issues in terms of accessibility for clinical trial sites, due to lack of transportation, site closures, and necessary quarantining measures. This also can cause confusion for patients as well, from lack of information in regards to the clinical trial organization and management, and as a result, their own health as well.
Some life science companies are working to overcome these challenges. PPD, a large global CRO, brings hope for conducting clinical trials in the pandemic while maintaining strong patient communication through their Accelerated Enrollment Solutions (AES) network unit. The unit works to transfer patients from other clinical trial research sites affected by the pandemic to their own, helping improve clarity of the organization of clinical trials for both researchers and patients. In order to do so, the AES project manager works with the involved parties including the sponsor, CRO, principal investigator and site staff to acquire their consent. Once consent is acquired, the patient is then informed of the site change, the AES team then confirms that the transfer is appropriate for the patient, and gathers all study records and contact information.
Afterwards, the AES staff conducts a digital consent visit with each patient to complete the transfer with the company and sponsor, ensuring full comprehension for the patient. Then, AES and the CRO configure the vendor systems and devices for continuity in the electronic trial master file. Following the new FDA and EMA guidances, the sponsor can organize a remote site initiation visit as well as regulatory filings at the same time. PPD has over 180 research sites across the US, China, India, Latin America, and EMEA, allowing not only greater opportunity for cross-border collaboration in the drug development process, but also helping to improve the health of patients’ globally.
Initiatives like PPD’s AES illustrates just one of many different innovative strategies to improve the drug development process, shifting from the traditional model to a new era of digitalization, where all facets of clinical trials are coordinated virtually and remotely. While life sciences companies are striving for efficiency and effectiveness through their innovative models for carrying on their researches and studies during the pandemic, it is equally as important for them to understand the uncertainty and pressure such quick transformation may bring to patients globally. To mitigate these stressors, implementation of inclusive patient communication strategies is necessary in maintaining a greater level of patient care and engagement, as well as making sure patients’ wants and needs are continuously monitored and met throughout the transition. In addition, this also helps ensure the patients’ rights in receiving adequate information and education about what they’re treatment.
For a clinical trial to be successful during these unprecedented times, even with an improved and innovative model, patients are a crucial component for success and trust of the treatment in the drug development process. With so much diversity in the world, with patients ranging in age, cultural background, dialects spoken, ethnicities, lifestyles, etc. life sciences company need to be thoughtful and in creating their hybrid strategies to better engage with target patient populations. Medical communication companies, like CSOFT Health Sciences, play a vital role for drug developers in helping engage and communicate with patients in an inclusive and culturally appropriate way in order to move towards a better and healthier world, together.
About CSOFT Health Sciences
CSOFT Health Sciences provides end-to-end medical translations for all phases of the product lifecycle, from pre-clinical to post-launch. We also specialize in market access consulting, medical writing, and CTD/eCTD submissions with the FDA, EMA, and NMPA. Our operations are compliant with ISO 17100 and certified in ISO 9001:2015 and ISO 13485:2016, ensuring our customized solutions meet the rigorous regulatory requirements of global submissions.
About CSOFT
CSOFT International is a leading provider of cross-border communications for enterprises seeking growth in global markets. Our expertise in localization, documentation, and branding encompasses a full range of end-to-end content and consulting services that we deliver in over 250 languages. With a focus in health sciences and smart technology, we work closely with our clients to deliver precision solutions to the challenges of engaging markets, consumers, and regulatory environments worldwide.
