As the trying times of the COVID-19 pandemic force all of us to adapt, one thing will never change: CSOFT’s dedication to you and your business needs.
We are proud to be involved in multiple initiatives and projects to facilitate solutions in the fight against COVID-19.
Fast turnaround is imperative for translation projects supporting the fight against COVID-19. CSOFT Health Sciences created the COVID-19 Emergency Response Team to ensure rapid delivery of urgent translation requests for clinical designs, protocols, and cross-border multi-center trial outcomes related to COVID-19 treatment and vaccine development.

A message from
our CEO & President,
Ms. Shunee Yee
“As we persevere through this challenging time, please know that we are always committed to go above and beyond to serve your needs in every capacity we can, at all times”
THE TOP 20 COUNTRIES FOR COVID-19 CLINICAL TRIAL DENSITY
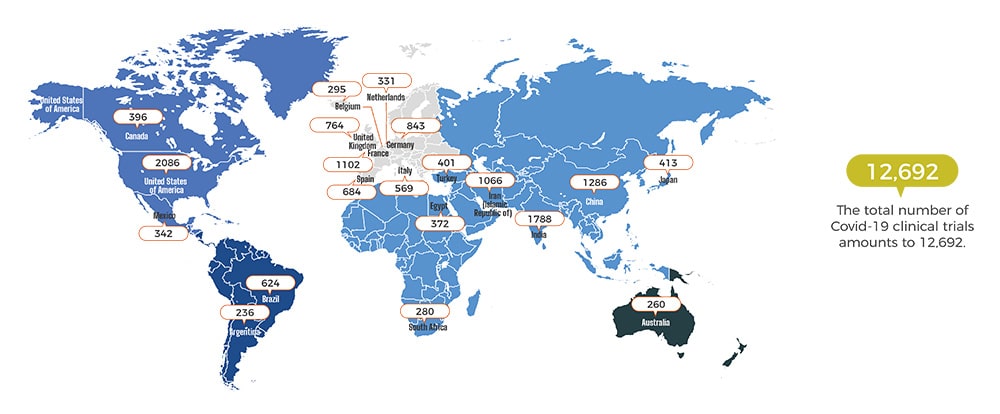
We are responding to the Coronavirus in full force. With dedicated medical translators in more than 186 countries, CSOFT’s CERT (COVID-19 Emergency Response Team) is equipped to address regional COVID-19 needs in every hard-hit region and resolve urgent translation requests in 250+ languages.
COVID-19 CSOFT RESPONSE INITIATIVE
Test Kit
- Marketing brochures
- Technical manuals
Vaccines/Drugs
- Clinical designs
- Human trial outcomes
- IND/NDA submission dossier
Patient Communication
- Cover the phone interpretation
Life Support Devices
- Operating procedures
- Technical manuals