China’s pharmaceutical market is the second-largest in the world, with multiple clinical trial sites and constant development of regulatory reforms, making it worth billions of dollars and a valuable investment for companies that can successfully gain Asia market access.

China Health Trends
Highly prevalent diseases
Patients statistics & demographics
Unmet clinical needs
Medical market value
Breakthroughs & Innovations
Clinical trials landscape
Drug discovery innovation
New technology & development
Hot topics

Regulatory Framework
NMPA
Chinese agency for regulating drugs and medical devices (formerly the China Food and Drug Administration or CFDA)
2019 China’s new drug administration law
Healthcare system
China regulatory reforms
Asia Market Access: Methodology
Research
- Data analytics & evaluation skills
- Product gap analysis
- Access to first-hand
market data & intelligence - Access to first-hand
market data & intelligence
Deliverables
- Market research reports
- Data summaries
- Proposals
- Comments & feedback
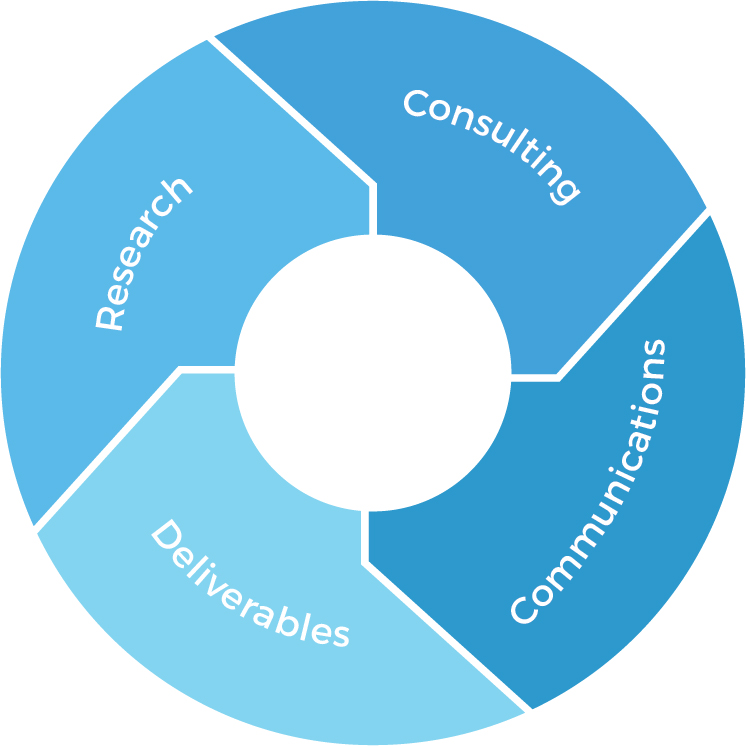
Asia Market Access Consulting
- Medical market
- Healthcare system
- Pharmaceutical regulations
- Latest updates and revisions to regulations
- Cultural and linguistic differences
Communications
- RA executives
- International marketing and sales team
- Key opinion leaders (KOLs)
- Local experts in NMPA regulations and clinical trials in China market