China is considered one of fastest-changing markets for global drug companies. As a result of changes in demographics, China’s regulatory and clinical landscapes have been undergoing fast and constant transformations.
China has an aging population, with 10 percent of it’s 1.38 billion citizens over 65. Additionally, the Chinese population accounts for a considerable percentage of worldwide cases of diabetes, hepatitis B and C, and cancers. Considering these facts, the medical needs facing the country in order to receive adequate treatment positions China as a key strategic clinical trial market for global drug developers.
Clinical trials are proven to be efficient and important for discovering new treatments for a variety diseases, as it helps reduce developing and redeveloping diseases. Foreign companies that are trying to conduct clinical trials in China are required to complete intensive paperwork and form partnerships with local Chinese companies. CSOFT’s strategist group guides global companies to provide a thorough understanding of the pharmaceutical industry in China and partners with experts to help them effectively engage with the Chinese market.

Advise Local qualified partners for conducting clinical trials / CROs
Strategize Clinical trial plans
Design Clinical trial proposals
Support CTA applications and other regulatory compliance matters
Monitor Safety and efficacy through the full product life cycle from clinical studies to post-approval commercialization
方法
Research
- Data analytics & evaluation skills
- Product gap analysis
- Access to first-hand
market data & intelligence - Access to first-hand
market data & intelligence
Deliverables
- Market research reports
- Data summaries
- Proposals
- Comments & feedback
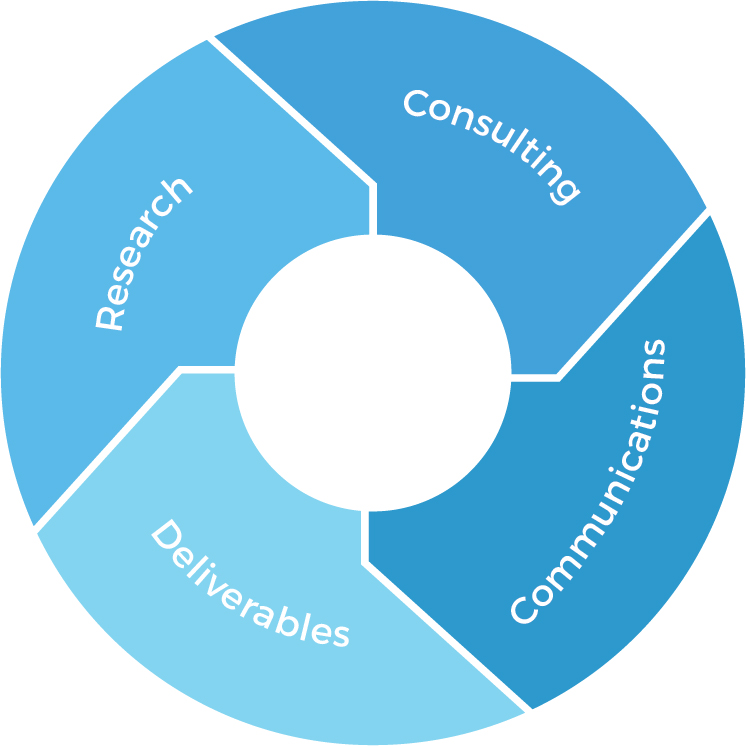
Consulting
- Medical market
- Healthcare system
- Pharmaceutical regulations
- Latest updates and revisions to regulations
- Cultural and linguistic differences
Communications
- RA executives
- International marketing and sales team
- Key opinion leaders (KOLs)
- Local experts in NMPA regulations and clinical trials in China market