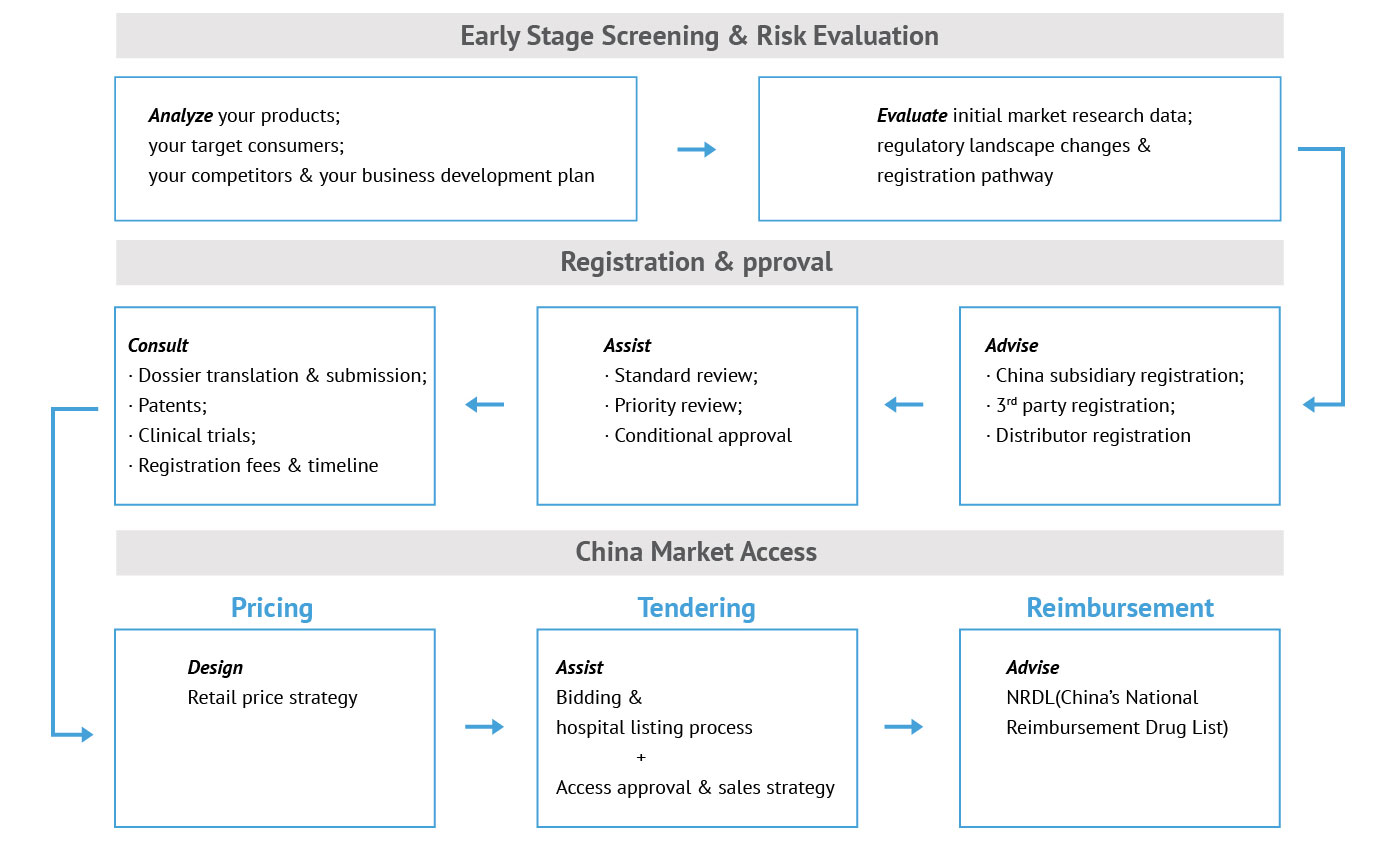
Before introducing new drugs into the Chinese market, companies must obtain approval from NMPA and create proper pricing and branding for the products. Our Asia regulatory strategy experts will work directly with your team to perform a comprehensive analysis and evaluate business opportunities versus risk factors.
The consultant team will give our clients specific guidelines on how to register their medical products and obtain faster approval from the NMPA. Afterward, we will design a plan to launch the product into China’s market and assist its implementation. Our team is dedicated to minimizing our clients’ risk while maximizing their business value and profits.
Enregistrement des produits médicaux
Clinical Trials in Asia: Methodology
Research
Data analytics & evaluation skills
Product gap analysis
Access to first-hand
market data & intelligence
Access to first-hand
market data & intelligence
Deliverables
Market research reports
Data summaries
Proposals
Comments & feedback
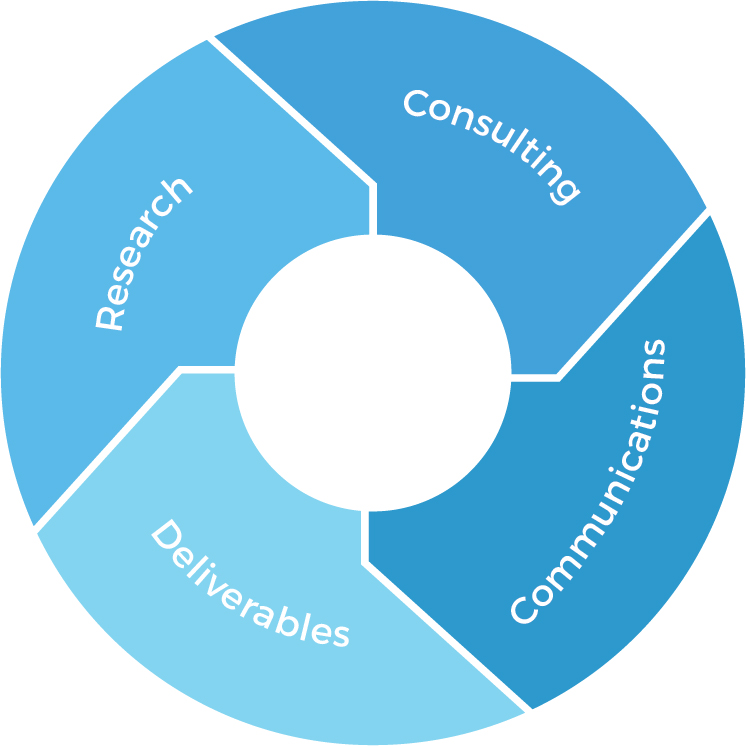
Consulting
Medical market
Healthcare system
Pharmaceutical regulations
Latest updates and revisions to regulations
Cultural and linguistic differences
Communications
RA executives
International marketing and sales team
Key opinion leaders (KOLs)
Local experts in NMPA regulations and clinical trials in China market